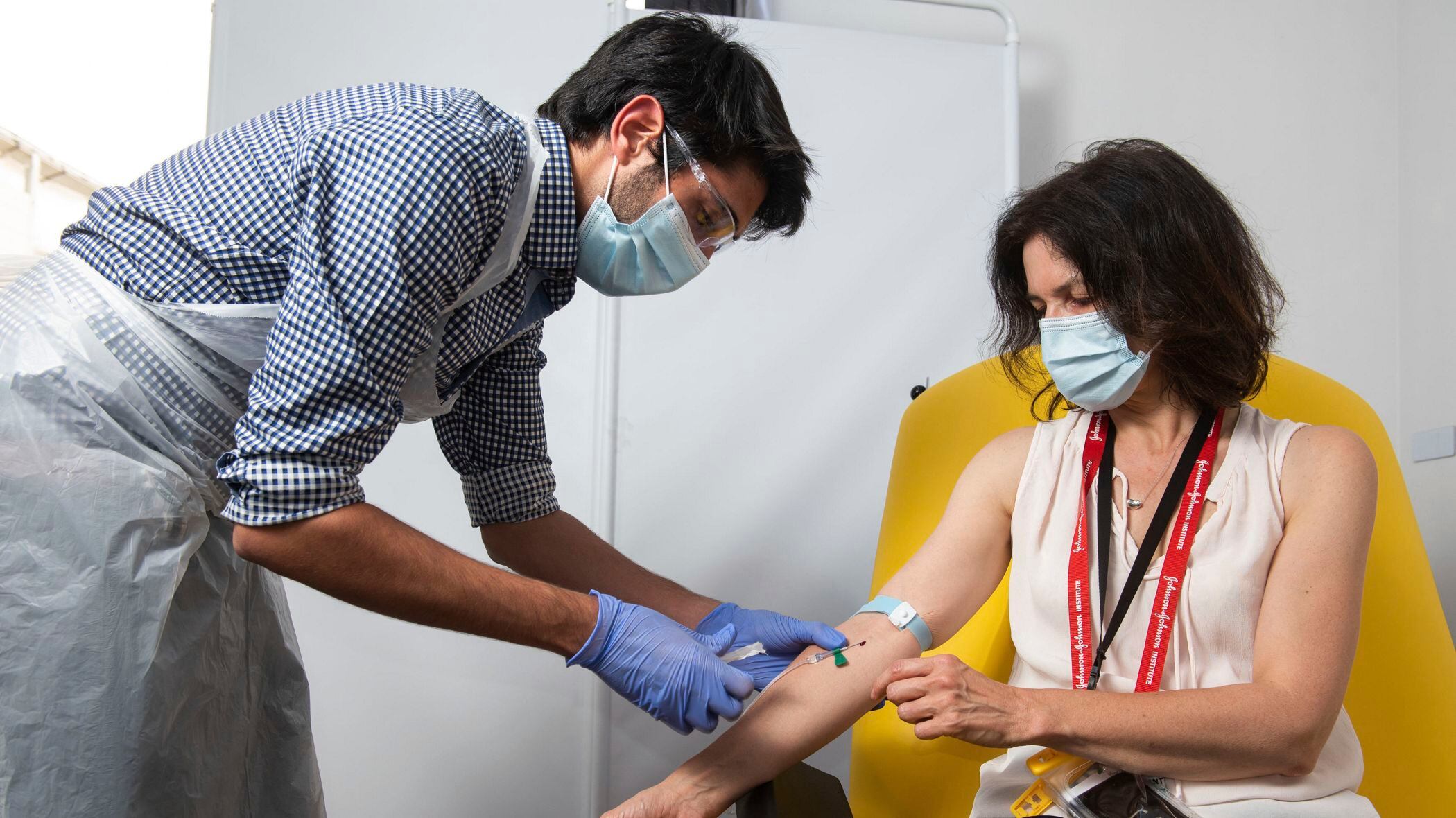
Industrial conglomerate 3M Company is partnering with the Massachusetts Institute of Technology to develop a cheaper, quicker COVID-19 test.
Right now, test results can take anywhere from a few hours to a week. Quest Diagnostics, for instance, said the average turnaround time at its labs is currently seven days.
3M's new test would function more like a pregnancy strip, a rapid paper-based test that could be mass-produced and quickly distributed at the point of care.
"In terms of patient experience, what we're looking to do is create that low-cost, highly-accurate, simple-to-use test that would be accessible to a lot of people," Dr. John Banovetz, chief technology officer for 3M, told Cheddar.
The antigen-based test, which looks at proteins on the virus, would offer a "preliminary view" of a patient's coronavirus status — though ideally, patients would follow up with a doctor, he added.
"Our goal would be a point-of-care test, so maybe it's taken in a doctor's office. Maybe you pick it up at the pharmacy," Banovetz said.
The goal is to start producing tests by the end of summer or early fall. Once manufacturing is rolling, 3M could produce millions of tests per day, according to the company.
As cases surge across the country and many anticipate a second wave in states where the infection rate has dropped off, calls for more testing are growing.
More than 41 million tests have been reported to the CDC, with a 9 percent positivity rate, but the U.S. continues to lag behind other countries by some measures.
In the meantime, the Trump administration is relying on the private sector to meet the demand for tests, putting pressure on companies such as 3M to come up with a solution.
"We're going as quick as we can right now," he said. "Our focus is trying to get that accuracy up and in a way that we know that we can mass-produce it. That's really where 3M can help contribute to this, our ability to be able to commercialize and bring to market new ideas and innovations."
Despite the expected arrival of COVID-19 vaccines in just a few weeks, it could take several months — probably well into 2021 — before things get back to something close to normal in the United States.
American Airlines is expanding its COVID-19 pilot pre-flight testing program to London in an effort to get more international flyers in the sky.
AstraZeneca reported that its vaccine is 90% effective and cheaper to distribute than vaccines from its competitors. Meanwhile, the U.S. is averaging 1,500 deaths per day according to Johns Hopkins.
Pharmaceutical company AstraZeneca said Monday that late-stage trials showed its coronavirus vaccine was up to 90% effective, giving public health officials hope they may soon have access to a vaccine that is cheaper and easier to distribute than some of its rivals.
Pfizer said Friday it is asking U.S. regulators to allow emergency use of its COVID-19 vaccine, starting the clock on a process that could bring limited first shots as early as next month and eventually an end to the pandemic -- but not until after a long, hard winter.
A satellite jointly developed by Europe and the United States being launched this weekend will greatly help scientists keep track of the rise in global sea levels, one of the most daunting effects of climate change, a senior official at the European Space Agency said Friday.
The use of mRNA technology in COVID-19 vaccines is groundbreaking for a number of reasons. However, there is a drawback to the vaccines: they have to be stored in brutally cold temperatures.
The U.S. decline in cigarette smoking could be stalling while the adult vaping rate appears to be rising, according to a government report released Thursday.
The only known white giraffe in the world has been fitted with a GPS tracking device to help protect it from poachers as it grazes in Kenya.
Pfizer said Wednesday that new test results show its coronavirus vaccine is 95% effective, is safe and also protects older people most at risk of dying.