By Matthew Perrone
U.S. officials granted full approval to a closely watched Alzheimer’s drug on Thursday, clearing the way for Medicare and other insurance plans to begin covering the treatment for people with the brain-robbing disease.
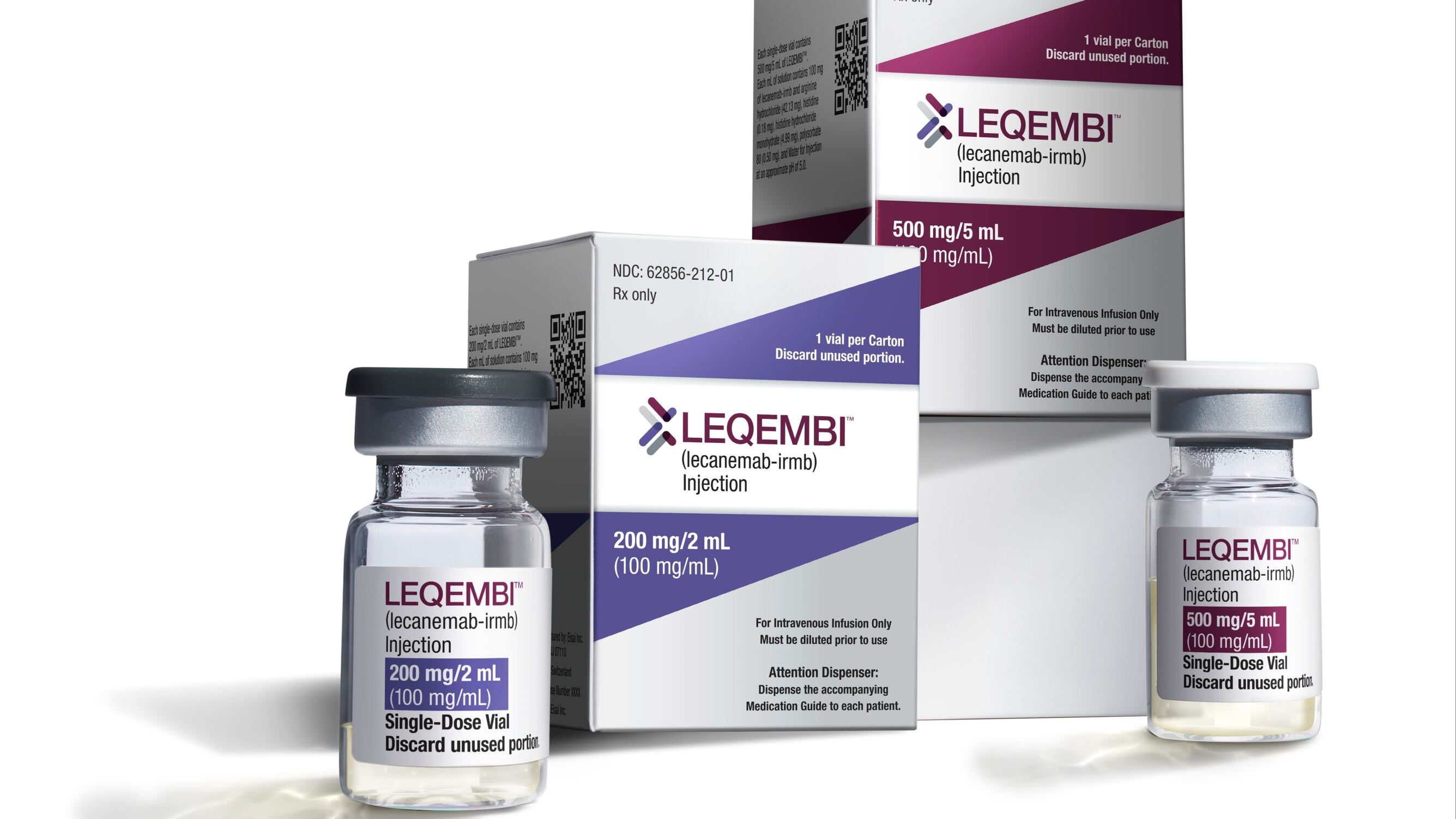
The Food and Drug Administration endorsed the IV drug, Leqembi, for patients with mild dementia and other symptoms caused by early Alzheimer's disease. It’s the first medicine that’s been convincingly shown to modestly slow Alzheimer’s cognitive decline.
Japanese drugmaker Eisai received conditional approval from the FDA in January based on early results suggesting Leqembi worked by clearing a sticky brain plaque linked to the disease.
The FDA confirmed those results by reviewing data from a larger, 1,800-patient study in which the drug slowed memory and thinking decline by about five months in those who got the treatment, compared to those who got a dummy drug.
“This confirmatory study verified that it is a safe and effective treatment for patients with Alzheimer’s disease," said FDA's neurology drug director, Teresa Buracchio, in a statement.
The drug's prescribing information will carry the most serious type of warning, indicating that Leqembi can cause brain swelling and bleeding, side effects that can be dangerous in rare cases. The label notes that those problems are seen with other plaque-targeting Alzheimer's drugs.
The process of converting a drug to full FDA approval usually attracts little attention. But Alzheimer’s patients and advocates have been lobbying the federal government for months after Medicare officials announced last year they wouldn’t pay for routine use of Leqembi until it received FDA’s full approval.
There were concerns that the cost of new plaque-targeting Alzheimer's drugs like Leqembi could overwhelm the program's finances, which provide care for 60 million seniors. Leqembi is priced at about $26,500 for a year’s supply of IVs every two weeks.
The vast majority of Americans with Alzheimer’s get their health coverage through Medicare. And private insurers have followed its lead by withholding coverage for Leqembi and a similar drug, Aduhelm, until they receive FDA's full endorsement. An FDA decision on full approval for Aduhelm is still years away.
Medicare administrator, Chiquita Brooks-LaSure, has made clear the program will immediately begin paying for the drug now that it has full FDA approval. But the government is also setting extra requirements.
Medicare recipients getting Leqembi must be enrolled in a federal registry to track the drug's real-world safety and effectiveness. The information will help advance “knowledge of how these drugs can potentially help people,” Medicare officials said.
Hospitals and medical clinics have also cautioned that it may take time to get people started on the drug.
Doctors need to confirm that patients have the brain plaque targeted by Leqembi before prescribing it. Nurses need to be trained to administer the drug and patients must be monitored with repeated brain scans to check for swelling or bleeding. The imaging and administration services carry extra costs for hospitals beyond the drug itself.
Eisai has told investors that about 100,000 Americans could be diagnosed and eligible to receive Leqembi by 2026. The drug is co-marketed with Cambridge, Massachusetts-based Biogen.
“We want to ensure that appropriate patients only are the ones that get this product,” said Alexander Scott, a vice president with Eisai.
Eisai studied the drug in people with early or mild disease who were evaluated using a scale measuring memory, thinking and other basic skills. After 18 months, those who got Leqembi declined more slowly — a difference of less than half a point on the scale — than participants who received a dummy infusion. Some Alzheimer's experts say that delay is likely too subtle for patients or their families to notice.
But federal health advisers said the difference could still be meaningful and recommended that FDA fully approve the drug at a public meeting in June.
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Science and Educational Media Group. The AP is solely responsible for all content.